|
Ozone Production and Destruction
|

|
| Creation
of Ozone. Courtesy of Distributed Active Archive
Center at NASA's Goddard Space Flight Center.
(Click
to launch movie.)
|
|
|
Stratospheric ozone is created and destroyed
primarily by ultraviolet radiation. The air in the stratosphere
is bombarded continuously with ultraviolet radiation from
the Sun. When high energy ultraviolet rays strike molecules
of ordinary oxygen (O2), they split the molecule into two
single oxygen atoms. The free oxygen atoms can then combine
with oxygen molecules (O2) to form ozone (03 molecules.
 |
| Destruction
of Ozone. Courtesy of Distributed Active Archive
Center at NASA's Goddard Space Flight Center.
(Click
to launch movie.)
|
|
|
O2 + UV light 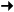 2 O
2 O
O + O2 + M 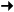 O3 + M (where M indicates conservation of energy
and momentum)
O3 + M (where M indicates conservation of energy
and momentum)
The same characteristic of ozone that makes
it so valuable, its ability to absorb a range of ultraviolet
radiation, also causes its destruction. When an ozone molecule
is exposed to ultraviolet energy it may break back into O2
and O. During dissociation the atomic and molecular oxygens
gain kinetic energy, which produces heat and causes an increase
in atmospheric temperature.
Ozone production is driven by UV radiation
of wavelengths less than 240 nm. Ozone dissociation typically
produces atomic oxygen that is stable when exposed to longer
wavelengths, up to 320 nm, and shorter wavelenghts of 400
to 700 nm. Longer wavelength photons penetrate more deeply
into the atmosphere, creating regions of ozone production
and destruction. When an ozone molecule absorbs even low energy
ultraviolet, it splits into an ordinary oxygen molecule and
a free oxygen atom.
O3 + UV, visible light
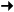 O + O2
O + O2
The free oxygen atom may then combine with an
oxygen molecule, creating another ozone molecule, or it may
take an oxygen atom from an existing ozone molecule to create
two ordinary oxygen molecules.
O + O2 -> O3
or O3 + O
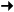 O2 + O2
O2 + O2
Processes of ozone production and destruction,
initiated by ultraviolet radiation, are often referred to
as "Chapman Reactions."
Most O3 destruction takes place
through catalytic processes rather than Chapman Reactions.
Ozone is a highly unstable molecule that readily donates its
extra oxygen molecule to free radical species such as nitrogen,
hydrogen, bromine, and chlorine. These compounds naturally
occur in the stratosphere, released from sources such as soil,
water vapor, and the oceans.
O3 + X
 XO + O2 ( where X may
be O, NO, OH, Br or Cl)
XO + O2 ( where X may
be O, NO, OH, Br or Cl)
Anthropogenic Destruction
Manufactured compounds are also capable of altering
atmospheric ozone levels. Chlorine, released from CFCs, and
bromine (Br), released from halons, are two of the most important
chemicals associated with ozone depletion. Halons are primarily
used in fire extinguishers. CFCs are used extensively in aerosols,
air conditioners, refrigerators, and cleaning solvents. Two
major types of CFCs are trichlorofluorocarbon (CFCl3), or
CFC-11, and dichlorodifluoromethane (CF2Cl2), or CFC-12. Trichlorofluorocarbon
is used in aerosols, while dichlorodifluoromethane is typically
used as a coolant.
CFCs were originally created to provide a substitute
for toxic refrigerant gases and reduce the occupational hazard
of compressor explosions. Near Earth's surface, chloroflourocarbons
are relatively harmless and do not react with any material,
including human skin. For 50 years they appeared to be the
perfect example of a benign technical solution to environmental
and engineering problems, with no negative side effects. While
CFCs remain in the troposphere they are virtually indestructible.
They are not water soluble and cannot even be washed out of
the atmosphere by rain. We now understand that the very quality
that made them seem so safe, their stability, is what makes
them so dangerous. CFCs remain in the troposhere for more
than 40 years before their slow migration to the stratosphere
is complete. Even if we were to end their production and use
at this very moment, they would continue to contribute to
ozone destruction far into the future.
In the stratosphere, high energy ultraviolet
radiation causes the CFC molecules to break down through photodissociation.
Atomic chlorine, a true catalyst for ozone destruction, is
released in the process. Chlorine initiates and takes part
in a series of ozone destroying chemical reactions and emerges
from the process unchanged. The free chlorine atom initially
reacts with an unstable oxygen containing compound, such as
ozone, to form chlorine monoxide (ClO).
Cl + O3
 ClO + O2
ClO + O2
The chlorine monoxide then reacts with
atomic oxygen to produce molecular oxygen and atomic chlorine.
The regenerated chlorine atom is then free to initiate a new
cycle.
ClO + O
 Cl + O2
Cl + O2
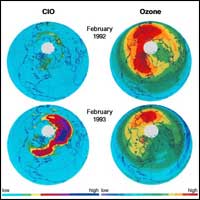
This destructive chain of reactions will continue
over and over again, limited only by the amount of chlorine
available to fuel the process.
 |
 |
|
|
Chlorine occurs naturally in the oceans. However,
the majority of chlorine in the atmosphere has originated
with man-made chemicals. Without the breakdown of manufactured
chlorofluorocarbons, there would be almost no chlorine in
the stratosphere. CFC-12 concentrations were less than 100
parts per trillion by volume when they were first measured
in the 1960s. Between 1975 and 1987, concentrations more than
doubled from less than 200 parts per trillion by volume to
more than 400 parts per trillion by volume. The amount of
chlorine in the stratosphere increased by a factor of 2 to
3. Scientists believe that continued buildup of CFCs could
lead to severe ozone loss worldwide. Ongoing studies are essential
to provide the necessary understanding of the causes of ozone
depletion. The history of CFCs demonstrates that human activities
can have an unexpected long-term effect on the environment.
Text, images
and videos courtesy of Distributed Active Archive Center at
NASA's Goddard Space Flight Center.
|