Astronomy 10: Lecture 5
Lecture 5
Tuesday, June 4th 2002. Reading: Cosmic Perspective Chapt. 3 and 4
The Scientific Method: Revisited
Key Points:
- Observation and Experiment required
- Hypothesis/model should explain and predict
- Hypothesis/model must be testable (disproof possible)
- Sharing of information is critical
- No knowledge is sacred or taboo
- Skeptical and Free Inquiry required
- Hypothesis/model becomes accepted as Theory only after it has passed all tests put to it. But if in the future new information comes to light that is inconsistent, then the Theory must be modified or rejected.
Further Scientific Priciples listed in Carl Sagan's Baloney Detection Kit
Pseudoscience and Nonscience
Pseudoscience: means to search for knowledge which on the surface appears to be scientific, but in reality breaks some of the most fundamental concepts of the scientific method. Examples: Astrology, UFO-ology, Paranormal Studies, Many 'New Age' philosophies and medical practices. Quite often what marks a pseudoscience is the blantant ignoring of evidence that does not support the basic hypothesis. A lack of rigorous experimentation is usually also quite lacking.
Nonscience: a means of seeking knowledge which is wholly unscientific. Does not use evidence or experiment. Requires no logic. Beliefs based upon faith, political conviction, tradition, or intuition. Examples: Religion, and mysticism. The idea that a God controls nature is not a scientific hypothesis. If it is not true, there is no way to proove it (the hypothesis is not disproovable).
"Nothing happens in contradiction to nature, only in contradiction to what we know of it."
- Special Agent Dana Scully, The X-Files
Astrology
In the beginning Astrology and Astronomy were one and the same.
The basic tenets of modern Astrology (Ptolemy's system) are that a person's character and destiny can be understood from the positions of the Sun, Moon, and Planets at the moment of his/her birth.
Astrologers use a chart called a horoscope and claim to be able to predict and explain the course of life and to help people, companies, and governments with decisions of great importance.
Skeptical Questions for Astrology:
- What is the likelihood that 1/12th of the world's population is having the same kind of day?
- Why is the moment of birth and not conception critical?
- If the mother's womb can keep out astrological influences until birth, can we do the same with a cubicle of steak?
- If astrologers are as good as they claim, why aren't they richer?
- Are all the horoscopes done before the discovery of the three outermost planets incorrect?
- Shouldn't we condemn astrology as a form of bigotry?
- Why do different schools of astrology disagree so strongly with each other?
- If the astrological influence is carried by a known force, why do the planets dominate?
- If astrological influence is carried by an unknown force, why is it independent of distance?
- If astrological influences don't depend on distance, why is there no astrology of stars, galaxies, quasars, etc.?
- Why can twins have different fates?
Scientific investigations of horoscope predictions find them to be no better than random guessing. Furthermore horoscopes tend not to be predictions at all but rather just advice. The advice is clothed in such vague language as to make an interpretation in hindsight seem as if the horoscope had actually predicted something.
Sun sign descriptions of personality are so general that any person can identify some of themselves in the description. Read a couple and figure out the percentage of the description that is actually fitting. Typically no better than 50%. Random guessing again.
Ancient Sun sign dates are plain wrong due to the precession of Earth's axis.
The Physical World
Energy
Energy is defined as a quantity that measures the ability of a system to do work or cause motion.
- Energy may neither be created or destroyed, but it can change form
- Metric unit of measure for Energy is Joules.

There are two kinds of mechanical energy
- Kinetic Energy: the energy of motion
for any object with mass, m, and velocity, v, its kinetic energy, KE, is given by
KE = 1/2 mv2
- Potential Energy: the amount of work that can potentially be done
This has no simple definition, it is defined in a mathematical way. For an object, with mass, m, under the gravitational attraction of another with mass, M, and a distance, r, away the potential energy, PE, is
PE = - GMm/r
The total mechanical energy, E, of a system is given by the sum of these two energies.
E = KE + PE
E is a constant and so KE and PE can only change in such a way that as one becomes smaller the other becomes larger by the same amount. In other words we can convert potential energy into kinetic energy and vice versa.
When you throw a ball up into the air, it initially has no Potential Energy, but has all Kinetic Energy. As the ball ascends it slows down, losing Kinetic Energy but gaining Potential Energy (the distance between the ball and Earth is increasing). When it reaches the top of its trajectory it has no velocity and therefore no Kinetic Energy, therefore it now has only Potential Energy. As the ball falls back to Earth, the situation reverses; Potential Energy converting to Kinetic Energy. All the while the value of E stays fixed.
Thermal Energy and Temperature
A Macroscopic measure of the energy contained within all the particles making up a system (solid, liquid, gas, etc.)
Temperature: The measure of the average Kinetic Energy of particles in a system.

Thermal Energy: The sum of all the individual kinetic energies of particles.
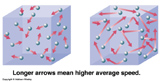
Matter
Fundamental Units of Matter: Atoms - from the Greek word meaning indivisible
Imagine dividing up a substance until you reach the level beyond which if you divided it further it would cease to be that substance. This what an Atom is. The fundamental unit of matter for the chemical elements. First suggested by Democritus.
While atoms are the fundamental units of the chemical elements (and molecules-- combinations of atoms -- are the fundamental units of other chemical substances), they are not actually fundamental. They have struture.
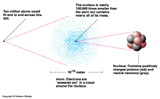
Atoms are made of Electrons (which buzz about in a cloud), Protons and Neutrons (huddled together in a nucleus). Electrons appear to be fundamental, but Protons and Neutrons appear to have structure. They are made up of elementary particles called Quarks.
The elementary particles are characterized by three basic properties: Mass, Charge, and Spin. For our purposes we'll only care about mass and charge. There are two kinds of charge positive and negative.
| Particle | Mass | Charge |
| Electron | 9.1094x10-31kg | -1.6022x10-19C |
| Proton | 1.6726x10-27kg | +1.6022x10-19C |
| Neutron | 1.6749x10-27kg | Neutral |
- Atoms are electrically neutral (equal numbers of protons and electrons).
- Chemical element is determined by number of protons in nucleus.
- Chemical properties are determined by the structure of the electron cloud.
- Losing or gaining electrons gives a net electrical charge and becomes known as an Ion, which will have different chemical properties than the atom.
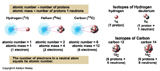
- Atoms with the same number of protons in the nucleus but different numbers of neutrons are called Isotopes. Effects nuclear properties of the atom. Can make it stable or unstable (which usually leads to radioactivity).
- Structure of the electron cloud depends on energies of the electrons.
Energy is Quantized. Electron may only have specific energies within an atom.

Matter Concepts
Density: The amount of mass per unit volume (g/cm3)
Phase: Depending on the average kinetic energy of individual atoms a collection of atoms will group together in various phases: Solid, Liquid, Gas, or Plasma.

Return to Class Notes Page









