Tuesday, June 18th 2002. Reading: Cosmic Perspective Chapters S4, 14, & 15
The other is the Uncertainty Priciple. Which says that the more we know about where a particle is located the less we know about its momentum. The more we know about its momentum, the less we know about its location.
How would we measure the location of an electron? Bounce light off it. But electrons are small and if you hit them with a with a photon of visible light which has a wavelength of 500 nm, you cannot pinpoint the location to within 500 nm (5,000 times the size of an atom). In order to pinoint the location to higher precision you need to use shorter wavelength photons. Like X-rays. But there is a problem. If you hit it with an X-ray you will give the electron a huge energy kick (X-rays have tons more energy than visible photons). That changes its momentum. So you gained info about the electrons location at the expense of its momentum.
Likewise to measure the momentum you would need to hit the electron with a low energy photon (say a microwave). But the wavelengths of a microwave are big (a few cm). You'll have no idea where the electron is to within a few cm.
This is not a technical issue. Again this is the way of nature.
Mathematically we can write the uncertainty principle as such:
The Uncertainty principle leads to the idea that an electron can only be described by a probability that it will be in a certain location with a certain momentum (or have a certain energy within a certain time). In an atom we know the momentum/energy of the electron fairly well so the location is smeared out into a wave.
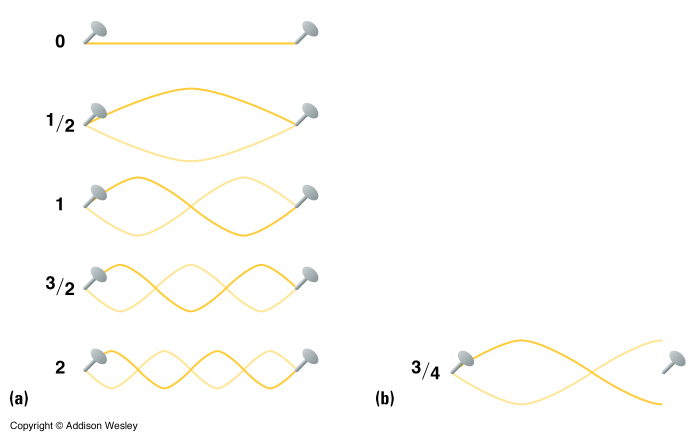
The interpretation of the electron as a wave within an atom helps us understand why it can only take on certain energy states. Think of the electron as a standing wave (a wave anchored on both ends which does not appear to travel along the distance but just vibrate in place). The electric force serves as the anchors for the electron to the nucleus. In a standing wave only certain wavelengths are allowed. So in the atom only certain wavelengths/energies are allowed for the electron to occupy.
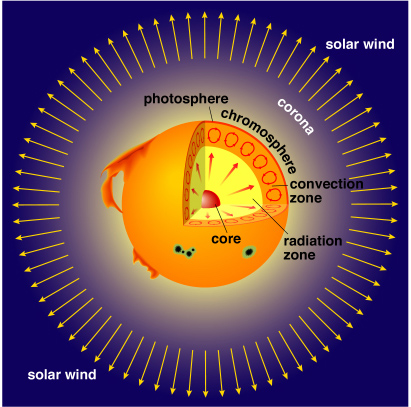
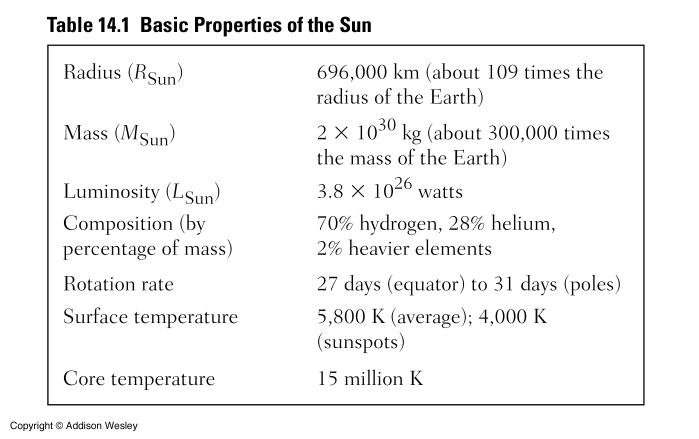
 Photosphere: The Sun's visible "surface". It's the lowest level of the Sun's atmosphere. Its diameter is about 1.4 x 106 km (0.5° in the sky). In the core the temperature is about Tcore = 1.5 x 107 K. From there the temperature steadily decreases out to the photosphere where the temperature is about T = 5,800 K.
Photosphere: The Sun's visible "surface". It's the lowest level of the Sun's atmosphere. Its diameter is about 1.4 x 106 km (0.5° in the sky). In the core the temperature is about Tcore = 1.5 x 107 K. From there the temperature steadily decreases out to the photosphere where the temperature is about T = 5,800 K.
By monitoring long lived sunspots one can see that the Sun spins on its axis about once per month. (It's not equal, the higher latitudes move slower than equatorial ones: differential rotation) |
 Chromosphere: Thin layer (104 km). T
Chromosphere: Thin layer (104 km). T |
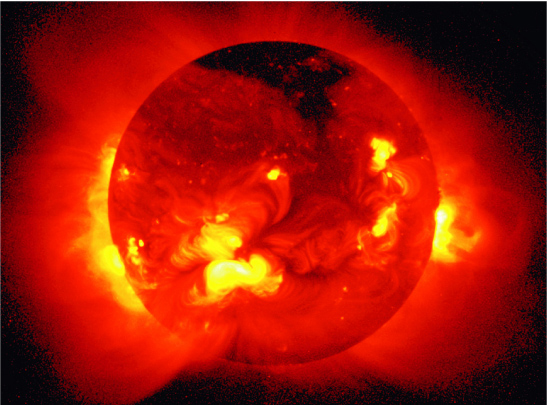 Corona: Large, low-density envelope (T
Corona: Large, low-density envelope (T |
Solar Wind: electrons and positive ions streaming from the Sun (v ![]() 500 km/s). Interacts with the planets' magnetic fields and with comets.
500 km/s). Interacts with the planets' magnetic fields and with comets.
Solar Flare: Violent release of energy from the Sun. (T ![]() 5 x 106 !). The matter from these can sometimes collide with Earth causing radio communication disruptions and fantastic auroras.
5 x 106 !). The matter from these can sometimes collide with Earth causing radio communication disruptions and fantastic auroras.
Prominence: more gentle eruption on Sun's surface. Loops of gas following magnetic fields lines above the Chromosphere, glowing red (T ![]() 104 K).
104 K).
Sunspots: dark blotches on the photosphere. Appear black or dark grey because they are around 2000 K cooler than their surroundings.
The Sun's surface (photosphere) is hot and opaque. That means that it acts as a thermal emitter. For thermal radiation there is a relationship between the amount of energy emitted per unit area per unit time, ![]() , and the Temperature, T. It's called the Stefan-Boltzmann Law:
, and the Temperature, T. It's called the Stefan-Boltzmann Law:
Where ![]() is the Stefan-Boltzmann constant (
is the Stefan-Boltzmann constant (![]() = 5.67 x 10-8 W m-2 K-4). So, we can see that for a thermal emitter a hot object emits much more energy than cold objects per unit area. This is why the temperature difference between the Sunspots and the surrounding Photosphere makes such a difference in brightness.
= 5.67 x 10-8 W m-2 K-4). So, we can see that for a thermal emitter a hot object emits much more energy than cold objects per unit area. This is why the temperature difference between the Sunspots and the surrounding Photosphere makes such a difference in brightness.
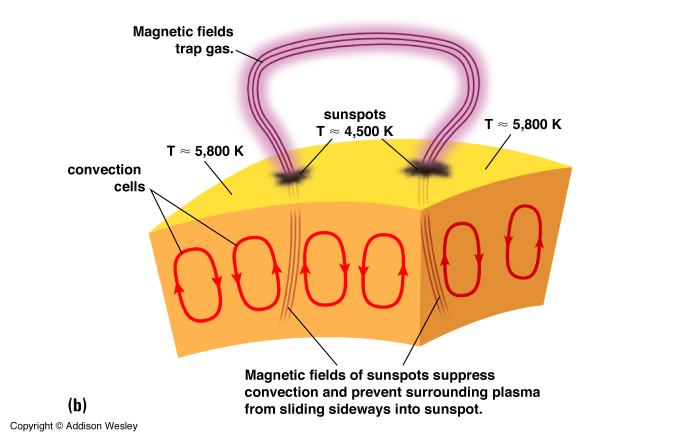 Sunspots are regions of strong magnetic fields that inhibit the rise of hot gas from below the photosphere.
Sunspots are regions of strong magnetic fields that inhibit the rise of hot gas from below the photosphere.
Solar Activity Cycle: Sunspots, prominences, and flares are numerous during peaks of an 11 year cycle. Previous maxima were in 1990, and 2001. The cycle is thought to be related to how the Sun rotates differentially and winds up and twists its magnetic field. The field becomes most twisted in 11 years and then breaks and reforms. The Solar cycle is thought to be related to climate changes on Earth. |
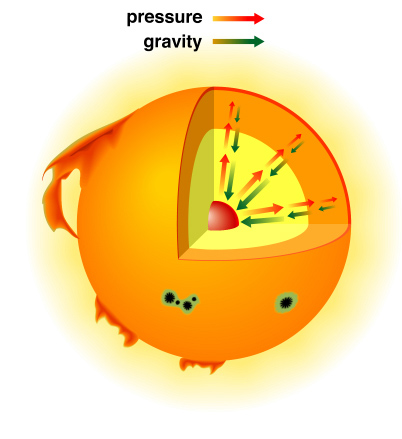 Gas exerts a pressure outward, which resists the force of gravity. Pressure is a force per unit area. You may recall from chemistry that it is proportional to density and Temperature
Gas exerts a pressure outward, which resists the force of gravity. Pressure is a force per unit area. You may recall from chemistry that it is proportional to density and Temperature (the perfect gas law: P = nkT, n is the number density of particles, k is another constant) Toward the center of the Sun temperature and density increases and so then does the outward pressure. The Sun is at a point where the internal pressure outward exactly balances the force of gravity inward. This point of mechanical balance is called Hydrostatic equilibrium. |
So pressure holds the Sun up. But what gives the particles their kinetic energy (Temperature) which allows such high pressures?
Could it be simple gravitational contraction, turning gravitational potential energy into kinetic energy? No? This could only provide the Sun with the needed energy for about 25 Million years. We know that the Solar System is more like 4.6 Billion years old. The early Protosun used gravitational contraction, but the Sun could not still be using it as a primary energy source.
What if the Sun were undergoing Chemical Burning? Is it "on fire"? No, that cannot be it either. Detailed calculations show that Chemical burning could provide the needed energy for only a few thousand years. Clearly not enough.
In the center of the Sun the conditions are so hot (T = 1.5 x 107 K) and dense that atoms have all of their electrons stripped away from them, they are completely ionized. Thus we have atomic nuclei (mostly hydrogen, single protons) all zipping to and fro in the core. Conditions are ripe for nuclear reactions which can produce a long-term energy source for the Sun.
For atomic nuclei the principle is the same; they interact with other atomic nuclei to make new configurations that have less energy than initially (more tightly bound) thus releasing energy in the reaction. (this reaction involves the strong nuclear force which is the strongest of the 4 fundamental forces of nature)
An Example:
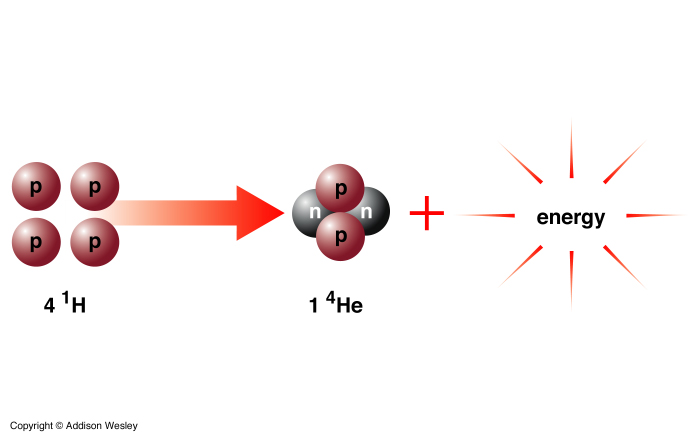
2He4 is more tightly bound than 41H1, so energy is emitted. It turns out that the Helium nucleus has less mass than the sum of 4 individual protons. In the reaction 0.7% of the mass of 41H1 is converted to energy:
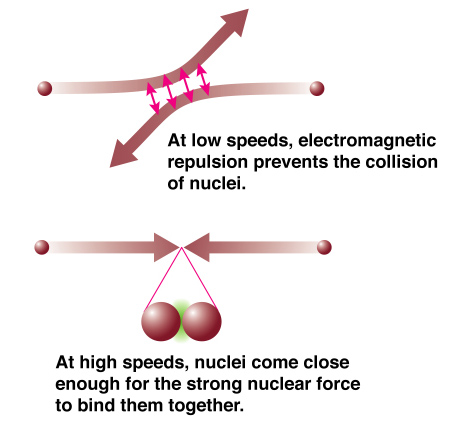 The reason we need hot temperatures and high densities for nuclear reactions to occur is that the strong nuclear force is a very short range force.
The reason we need hot temperatures and high densities for nuclear reactions to occur is that the strong nuclear force is a very short range force.
The electric force makes protons repel one another. The nuclei (protons) have to be moving very fast and have enough near collisions with each other so that they can get close enough for the strong nuclear force to take effect. Once it does it is far, far stronger than the electrical repulsion that the protons feel. |
Iron (Fe) - 26 protons - has the most tightly bound nucleus, so fusion of nuclei up to Fe will release energy.
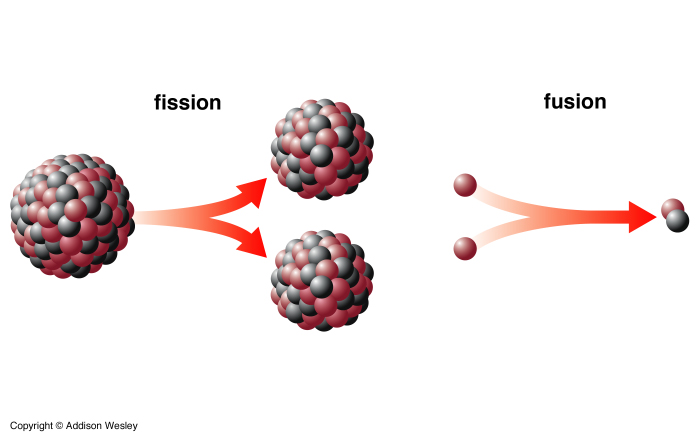 Fission of heavier nuclei into lighter one also releases energy, for example 92U238, but these nuclei are very rare and don't contribute any energy to the star.
Fission of heavier nuclei into lighter one also releases energy, for example 92U238, but these nuclei are very rare and don't contribute any energy to the star.
Nuclear power plants and the orginal atomic bombs use fission reactions. The so-called H-bomb is actually a fission bomb which implodes to cause a fusion reaction that subsequently explodes. Fusion on Earth for power has yet to be successfully achieved. Reactions still require more energy to get them going that we get back from them. When we eventually work out the kinks Fusion energy will solve the World's energy problem. It produces tons of energy and the bi-products are not radioactive. |
By mass, Sun is 70% H, 28% He, and 2% heavier elements: Lot's of raw material to fuse ! The Sun can fuse hydrogen into helium for about 10 Billion years before running out of fuel. So the Sun is a middle-aged star, 5 Billion down, another 5 billion to go.
NOTE: The photons produced in the core take millions of years to leak out from the core out to the photosphere where they then stream away freely into cold, dark space.

Solar Neutrinos:
Several reactions can occur depending on the temperature of the core. Net Effect: conversion of 4 protons to a Helium nucleus (2 protons + 2 neutrons) plus energy.
The reaction that converts 4 protons to a He nucleus involves the conversion of protons into neutrons.
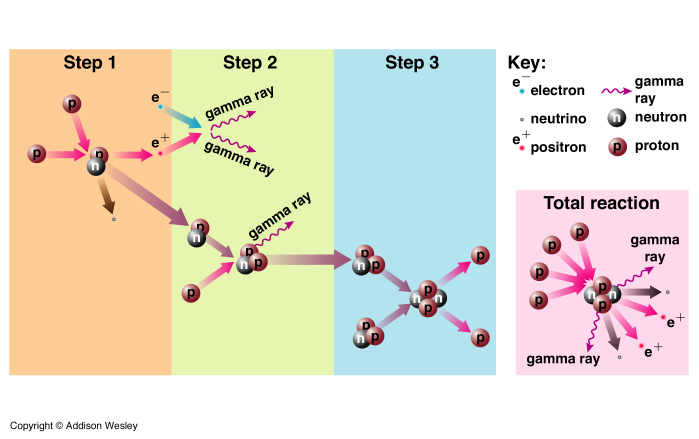
neutrinos (![]() ) have very little (if any) mass, and travel near the speed of light. They hardly react with anything. They can escape directly from the core, unlike photons. Hence we can observe them to probe directly the conditions in the core of the Sun.
) have very little (if any) mass, and travel near the speed of light. They hardly react with anything. They can escape directly from the core, unlike photons. Hence we can observe them to probe directly the conditions in the core of the Sun.
To do this we build giant tanks filled with a liquid compound akin to cleaning solution and put them far underground. Since neutrinos hardly react to anything we expect to detect only 1 neutrino per day interacting with the liquid in the tank! We actually detect about a third fewer!
Most probable solution is that neutrinos have some (non-zero) mass which allows them to mutate from one kind of neutrino to another. We can only currently detect electron neutrinos, which are those produced in the center of the Sun.
Return to Class Notes Page